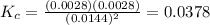Chemistry, 27.06.2020 15:01 cynthiafchs9203
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibrium concentration of H2 is 0.0028M, then what is the equilibrium constant of the reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibr...
Questions



Spanish, 19.03.2021 20:50

Mathematics, 19.03.2021 20:50


Mathematics, 19.03.2021 20:50



Mathematics, 19.03.2021 20:50



Computers and Technology, 19.03.2021 20:50

Advanced Placement (AP), 19.03.2021 20:50

Social Studies, 19.03.2021 20:50

Advanced Placement (AP), 19.03.2021 20:50

Computers and Technology, 19.03.2021 20:50




![K_{c} =\frac{[H_{2}][F_{2}] }{[HF]^2}](/tpl/images/0695/8873/96c9f.png)