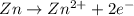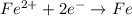Chemistry, 27.06.2020 09:01 wsdafvbhjkl
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this electron gain mean for iron?
A
It is neutralized
B.
It is oxidized
C. It is reduced
D.
It has dissolved.
E
It has precipitated

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this...
Questions


Biology, 12.07.2019 19:00

Mathematics, 12.07.2019 19:00

Mathematics, 12.07.2019 19:00

English, 12.07.2019 19:00

Social Studies, 12.07.2019 19:00




Mathematics, 12.07.2019 19:00



Mathematics, 12.07.2019 19:00






History, 12.07.2019 19:00

Mathematics, 12.07.2019 19:00