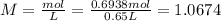Chemistry, 26.06.2020 17:01 villafana36
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16 g/mol, what is the molarity of this solution? answer options are 0.0106 M C6H12O6 0.0195 M C6H12O6 1.07 M C6H12O6 1.92 M C6H12O6
need help ASAP
will mark brainlest

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16...
Questions

Advanced Placement (AP), 02.11.2020 23:00

Mathematics, 02.11.2020 23:00

Social Studies, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00

Geography, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00



History, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00



Geography, 02.11.2020 23:00

Chemistry, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00


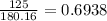 mol de glucosa
mol de glucosa