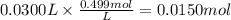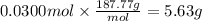Chemistry, 27.06.2020 05:01 viktoria1198zz
Aqueous solutions of copper (II) bromide and silver (1) acetate react to form solid
silver (1) bromide and aqueous copper (II) acetate according to the UNBALANCED
reaction below.
CuBr2 (aq) + AGCH3CO2 (aq)
-
AgBr (s) + Cu(CH3CO2)2 (aq)
How many grams of silver (1) bromide will form if 30.0 mL of 0.499 M copper (II)
bromide react with excess silver (1) acetate?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Aqueous solutions of copper (II) bromide and silver (1) acetate react to form solid
silver (1) brom...
Questions


Medicine, 16.04.2020 07:00







Mathematics, 16.04.2020 07:00



Mathematics, 16.04.2020 07:00

Mathematics, 16.04.2020 07:00


English, 16.04.2020 07:00

Biology, 16.04.2020 07:00



History, 16.04.2020 07:01

Mathematics, 16.04.2020 07:01