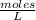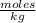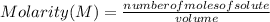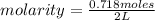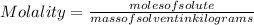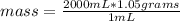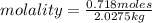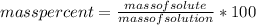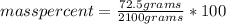Chemistry, 26.06.2020 16:01 kyaslaven9791
An aqueous KNO3 solution is made using 72.5 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molarity, molality, and mass percent of the solution. (Assume the density of 1.05 g/mL for the solution.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
An aqueous KNO3 solution is made using 72.5 g of KNO3 diluted to a total solution volume of 2.00 L....
Questions

Spanish, 30.11.2021 20:10

Social Studies, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Physics, 30.11.2021 20:10

Social Studies, 30.11.2021 20:10




Mathematics, 30.11.2021 20:10



Mathematics, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Physics, 30.11.2021 20:10



Physics, 30.11.2021 20:10



Social Studies, 30.11.2021 20:10