Chemistry, 26.06.2020 15:01 gwendallinesikes
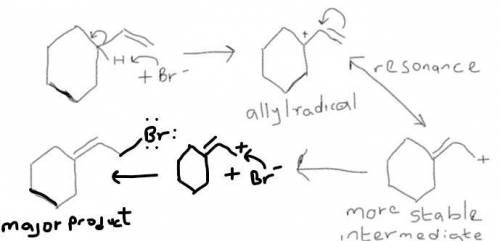
When vinylcyclohexane is treated with in dichloromethane, the major product is (2-bromo ethylidene)cyclohexane . Account for the formation of this product by drawing the structure of the most stable radical intermediate. Include all valence lone pairs in your answer. Include all valence radical electrons in your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
When vinylcyclohexane is treated with in dichloromethane, the major product is (2-bromo ethylidene)c...
Questions

Mathematics, 01.06.2021 21:40


Mathematics, 01.06.2021 21:40



Mathematics, 01.06.2021 21:40

History, 01.06.2021 21:40

English, 01.06.2021 21:40

Arts, 01.06.2021 21:40

Computers and Technology, 01.06.2021 21:40


Mathematics, 01.06.2021 21:40


Mathematics, 01.06.2021 21:40



Mathematics, 01.06.2021 21:40


Mathematics, 01.06.2021 21:40

Advanced Placement (AP), 01.06.2021 21:40