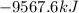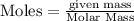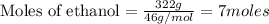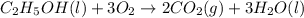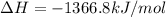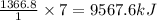Chemistry, 26.06.2020 15:01 nehaljay1883
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g)⟶2CO2(g)+3H2O(l)ΔH∘ c=−1366.8kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g...
Questions











Mathematics, 13.08.2021 04:20

Mathematics, 13.08.2021 04:20



Mathematics, 13.08.2021 04:20