Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
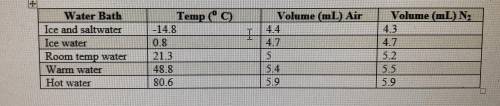
If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe...
Questions

Social Studies, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

SAT, 22.02.2021 14:00



Medicine, 22.02.2021 14:00


Mathematics, 22.02.2021 14:00

Physics, 22.02.2021 14:00


English, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00



Mathematics, 22.02.2021 14:00