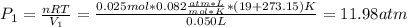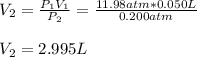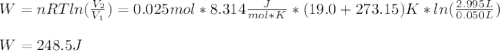A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature expansion against a constant pressure of 0.200 atm. If the gas is allowed to expand unchecked until its pressure is equal to the external pressure, what would its final volume be before it stopped expanding, and what would be the work done by the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature e...
Questions






Mathematics, 20.09.2020 02:01

Biology, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01




History, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01


Computers and Technology, 20.09.2020 02:01

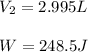
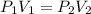
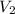 by firstly computing the initial pressure:
by firstly computing the initial pressure: