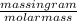Chemistry, 24.06.2020 19:01 SketchWasTaken
What is the mass of 0.25mol of NO3- ions/nitrate ions

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2

Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
What is the mass of 0.25mol of NO3- ions/nitrate ions...
Questions

English, 20.05.2020 23:00


Mathematics, 20.05.2020 23:00

English, 20.05.2020 23:00


History, 20.05.2020 23:00

Mathematics, 20.05.2020 23:00



Mathematics, 20.05.2020 23:01

Mathematics, 20.05.2020 23:01

Mathematics, 20.05.2020 23:01

Mathematics, 20.05.2020 23:01



Social Studies, 20.05.2020 23:01


History, 20.05.2020 23:01

Mathematics, 20.05.2020 23:01

Chemistry, 20.05.2020 23:01