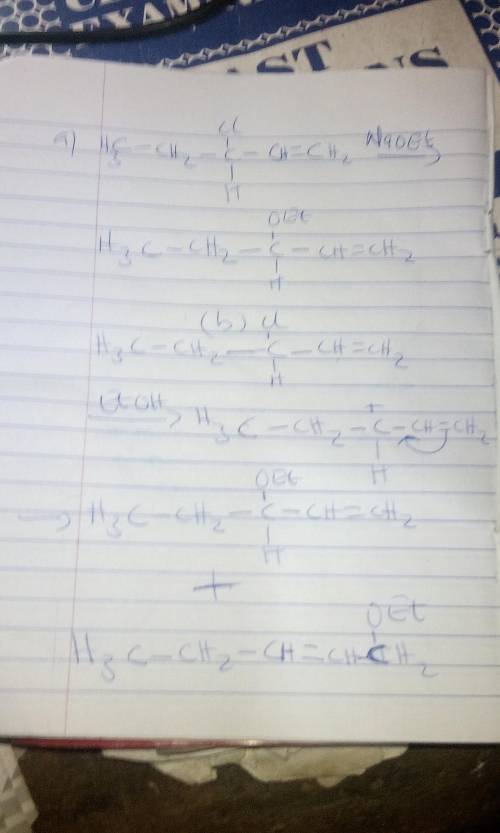Chemistry, 26.06.2020 16:01 tamyrareaves12
3-Chloro-1-pentene reacts with sodium ethoxide in ethanol to produce 3-ethoxy-1-pentene. The reaction is second order, first order in 3-chloro-1-pentene and first order in sodium ethoxide. In the absence of sodium ethoxide, 3-chloro-1-pentene reacts with ethanol to produce both 3-ethoxy-1-pentene and 1-ethoxy-2-pentene. The first reaction proceeds via an mechanism. The stereochemistry of the product is . The second reaction proceeds via an mechanism. The stereochemistry of 3-ethoxy-1-pentene is . The stereochemistry of 1-ethoxy-2-pentene is . For the second reaction, draw the structure of the intermediate's resonance contributor that leads to the formation of 3-ethoxy-1-pentene.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
3-Chloro-1-pentene reacts with sodium ethoxide in ethanol to produce 3-ethoxy-1-pentene. The reactio...
Questions


Mathematics, 29.08.2020 23:01

Mathematics, 29.08.2020 23:01





Mathematics, 29.08.2020 23:01

History, 29.08.2020 23:01





Computers and Technology, 29.08.2020 23:01


History, 29.08.2020 23:01