
Chemistry, 25.06.2020 07:01 Angeldelissa
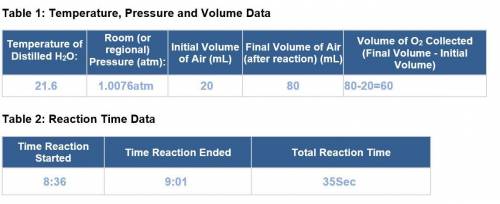
1. Calculate the number of moles of O2 produced using the ideal gas law. Then, use this value to calculate the number of moles of hydrogen peroxide you began the experiment with. HINT: Use the balanced equation provided in the lab introduction. 2. Calculate the number of moles of hydrogen peroxide you would have if you used 5 mL of a pure hydrogen peroxide solution. HINT: The density of hydrogen peroxide is 1.02 g/mL. 3. Determine the percentage of hydrogen peroxide in your solution. 1.02 g/mL * 5 mL = 5.1g / 34 g = 0.15 mol 4. Was the calculated percentage of hydrogen peroxide close to the same as the percentage on the label (3%)? Calculate percent error of your value.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 09:00
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

You know the right answer?
1. Calculate the number of moles of O2 produced using the ideal gas law. Then, use this value to cal...
Questions




Biology, 09.12.2020 05:40


Chemistry, 09.12.2020 05:40

Mathematics, 09.12.2020 05:40


Arts, 09.12.2020 05:40



Computers and Technology, 09.12.2020 05:40


Health, 09.12.2020 05:40



Biology, 09.12.2020 05:40


Mathematics, 09.12.2020 05:40

History, 09.12.2020 05:40




