
Chemistry, 25.06.2020 07:01 khristaviaaa
Calculate the pH of the acetate buffer CH3COOH + CH3COONa, containing 0.01 of each of the compound. Ka= 1.8×10^-5

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 14:30
The first supersonic flight was in 1947. it was just above the speed of sound. which altitude would you expect captain yeager to have used for his flight
Answers: 3

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1
You know the right answer?
Calculate the
pH of the acetate buffer CH3COOH + CH3COONa, containing 0.01 of each of the compound....
Questions


English, 03.02.2020 21:58


Biology, 03.02.2020 21:58

Computers and Technology, 03.02.2020 21:58

Mathematics, 03.02.2020 21:58


Biology, 03.02.2020 21:58

Mathematics, 03.02.2020 21:58

Biology, 03.02.2020 21:58

Mathematics, 03.02.2020 21:58



Mathematics, 03.02.2020 21:58







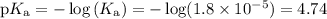
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = &4.74 +\log \left(\dfrac{0.01}{0.01}\right )\\\\& = & 4.74 + \log1.0 \\& = & 4.74 +0.0\\& = &4.7 \\\end{array}\\\text{The pH is $\large \boxed{\textbf{4.7}}$}](/tpl/images/0693/9328/22ba3.png)


