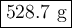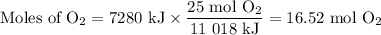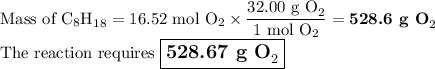Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
A sample of octane undergoes combustion according to the equation 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O...
Questions


Social Studies, 07.12.2020 20:50


History, 07.12.2020 20:50



Mathematics, 07.12.2020 20:50





Advanced Placement (AP), 07.12.2020 20:50

Mathematics, 07.12.2020 20:50

Mathematics, 07.12.2020 20:50




Mathematics, 07.12.2020 20:50


English, 07.12.2020 20:50