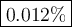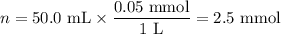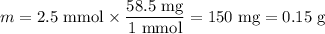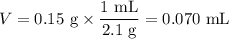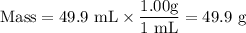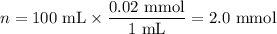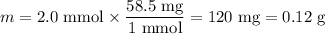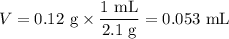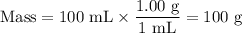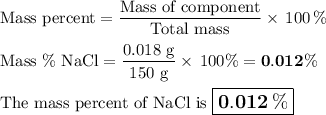Chemistry, 24.06.2020 06:01 maevemboucher78
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaCl solution. What is the mass percent of NaCl in the final solution?
Assume the volumes are additive and their densities 21 g/mL. The molar mass of
NaCl is 58.5 g/mol. (10 points)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1


You know the right answer?
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaC...
Questions




Mathematics, 10.12.2020 01:00




Mathematics, 10.12.2020 01:00



Mathematics, 10.12.2020 01:00


English, 10.12.2020 01:00


History, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Biology, 10.12.2020 01:00


History, 10.12.2020 01:00