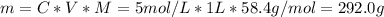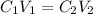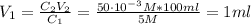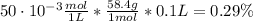Chemistry, 21.06.2020 04:57 trevinojazzy8625
You have a 5 M NaCl stock solution. (NaCl F. W. is 58.4) a) How much NaCl (in g) do you need to make 1 liter of the 5 M stock solution? b) How much stock solution will you need if you want to make 100 ml of 50 mM NaCl? c) What is the % concentration of a 50 mM solution of NaCl?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
You have a 5 M NaCl stock solution. (NaCl F. W. is 58.4) a) How much NaCl (in g) do you need to make...
Questions

Medicine, 20.07.2019 03:10



Engineering, 20.07.2019 03:10







Engineering, 20.07.2019 03:20