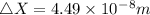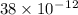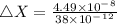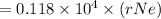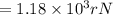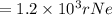An atom of helium has a radius = and an average speed in the gas phase at of . . Suppose the speed of a helium atom at has been measured to within . Calculate the smallest possible length of box inside of which the atom could be known to be located with certainty. Write your answer as a multiple of and round it to significant figures. For example, if the smallest box the atom could be in turns out to be times the radius of an atom of helium, you would enter "" as your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
An atom of helium has a radius = and an average speed in the gas phase at of . . Suppose the speed o...
Questions



Mathematics, 09.06.2020 21:57



Mathematics, 09.06.2020 21:57


Mathematics, 09.06.2020 21:57

History, 09.06.2020 21:57



Mathematics, 09.06.2020 21:57

Biology, 09.06.2020 21:57

Mathematics, 09.06.2020 21:57

Social Studies, 09.06.2020 21:57

Mathematics, 09.06.2020 21:57

Mathematics, 09.06.2020 21:57


Mathematics, 09.06.2020 21:57

English, 09.06.2020 21:57

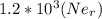
 m/s
m/s

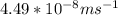
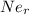 (radius of Neon) i.e.
(radius of Neon) i.e. 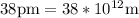
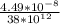
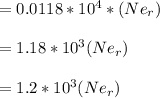

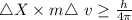 ....... (i)
....... (i)