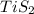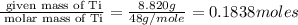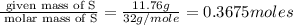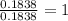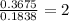Chemistry, 20.06.2020 23:57 saraaaaaaaa20
A student wants to determine the empirical formula for titanium sulfide (TixSy). To do so, she reacted titanium with excess sulfur in a crucible, and recorded the following data: Mass of Crucible: 11.120 g Mass of titanium used: 8.820 g Mass of Crucible and product: 31.700 g what is the empirical formula of titanium sulfide based on her experiment?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
A student wants to determine the empirical formula for titanium sulfide (TixSy). To do so, she react...
Questions

Mathematics, 16.10.2019 05:40


Mathematics, 16.10.2019 05:40


Mathematics, 16.10.2019 05:40

Computers and Technology, 16.10.2019 05:40

English, 16.10.2019 05:40

History, 16.10.2019 05:40


Mathematics, 16.10.2019 05:40


Mathematics, 16.10.2019 05:40





History, 16.10.2019 05:40

Mathematics, 16.10.2019 05:40


Biology, 16.10.2019 05:40