
Chemistry, 20.06.2020 22:57 deojahnaeb37
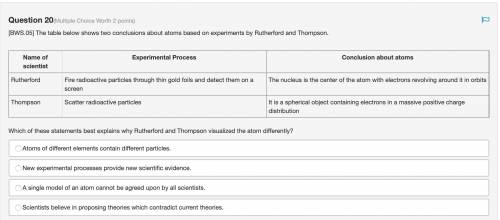
The table below shows two conclusions about atoms based on experiments by Rutherford and Thompson. Which of these statements best explains why Rutherford and Thompson visualized the atom differently? Atoms of different elements contain different particles. New experimental processes provide new scientific evidence. A single model of an atom cannot be agreed upon by all scientists. Scientists believe in proposing theories which contradict current theories.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Asample of rose gold is: 12.0 g gold, 5.0g silver, and 7.0 g copper. what is the percent copper in the sample?
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
The table below shows two conclusions about atoms based on experiments by Rutherford and Thompson. W...
Questions

Social Studies, 13.01.2020 12:31

Physics, 13.01.2020 12:31


Mathematics, 13.01.2020 12:31

History, 13.01.2020 12:31




Health, 13.01.2020 12:31

Mathematics, 13.01.2020 12:31

English, 13.01.2020 12:31

Mathematics, 13.01.2020 12:31





Mathematics, 13.01.2020 12:31

Mathematics, 13.01.2020 12:31


Mathematics, 13.01.2020 12:31



