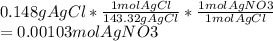Chemistry, 19.06.2020 17:57 kittenface3428
To lift fingerprints from a crime scene, a solution of silver nitrate is sprayed on a surface to react with the sodium chloride left behind by perspiration. What is the molarity of a silver nitrate solution if 42.8 mL of it reacts with excess sodium chloride to produce 0.148g of precipitate according to the following reaction?
AgNO3(aq)+NaCl(aq) --> AgCl(s)+NaNO3(aq)
A) 2.41 x 10^-2 M
B) 0.0229 M
C) 6.66 x 10^-2 M
D) 3.2 x 10^-3 M
E) 2.29 x 10^2 M

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
To lift fingerprints from a crime scene, a solution of silver nitrate is sprayed on a surface to rea...
Questions



Chemistry, 18.10.2019 07:00







Mathematics, 18.10.2019 07:00


Mathematics, 18.10.2019 07:00

History, 18.10.2019 07:00


Chemistry, 18.10.2019 07:00



Mathematics, 18.10.2019 07:00

Mathematics, 18.10.2019 07:00