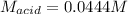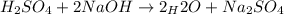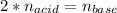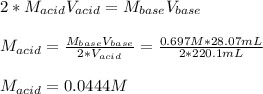Suppose you are titrating a sulfuric acid solution of unknown concentration with a sodium hydroxide solution according to the equation H 2 S O 4 + 2 N a O H ⟶ 2 H 2 O + N a 2 S O 4 If you require 28.07 mL of 0.697 M NaOH solution to titrate 220.1 mL of H 2 SO 4 solution, what is the concentration of the H 2 SO 4 solution? Type

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2


Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
Suppose you are titrating a sulfuric acid solution of unknown concentration with a sodium hydroxide...
Questions

Physics, 12.11.2020 21:50



Mathematics, 12.11.2020 21:50

Physics, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50



Mathematics, 12.11.2020 21:50




Biology, 12.11.2020 21:50


Health, 12.11.2020 21:50


English, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

History, 12.11.2020 21:50