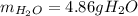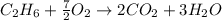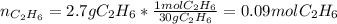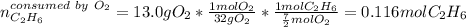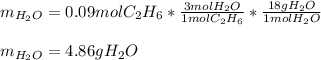Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 2.7 g of ethane is mixed with 13.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions

History, 13.10.2020 02:01





Arts, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01




Mathematics, 13.10.2020 02:01



Mathematics, 13.10.2020 02:01

Law, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01