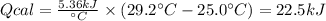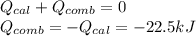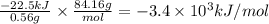Chemistry, 20.06.2020 12:57 stormserena
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant volume) calorimeter, with the products being carbon dioxide and liquid water. The calorimeter's heat capacity is 5.36 kJ °C-1. If the temperature inside the calorimeter increased from 25.0 °C to 29.2 °C, determine ΔrH for this reaction in kJ mol-1 (with respect to C6H12) at 298 K. Do not worry about how realistic the final answer is. You have 5 attempts at this question. TIP: To report an answer in scientific notation, enter it using the format "2.3E4", which means "2.3 x 104" (without the quotation marks)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant...
Questions

Biology, 23.07.2019 17:10

Business, 23.07.2019 17:10

Biology, 23.07.2019 17:10

Mathematics, 23.07.2019 17:10

Chemistry, 23.07.2019 17:10



History, 23.07.2019 17:10

Business, 23.07.2019 17:10

Social Studies, 23.07.2019 17:10

Business, 23.07.2019 17:10

Biology, 23.07.2019 17:10

History, 23.07.2019 17:10

History, 23.07.2019 17:10

History, 23.07.2019 17:10

History, 23.07.2019 17:10


Chemistry, 23.07.2019 17:10

Mathematics, 23.07.2019 17:10

Mathematics, 23.07.2019 17:10

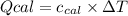
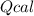 : heat absorbed by the calorimeter
: heat absorbed by the calorimeter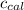 : heat capacity of the calorimeter
: heat capacity of the calorimeter : change in the temperature
: change in the temperature