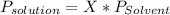Chemistry, 20.06.2020 05:57 kodiebclay
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H8O4, a nonvolatile, nonelectrolyte (MW = 180.1 g/mol), must be added to 219.0 grams of diethyl ether to reduce the vapor pressure to 458.5 mm Hg ? diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

You know the right answer?
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H...
Questions


Mathematics, 31.03.2021 15:30

Geography, 31.03.2021 15:30

Physics, 31.03.2021 15:30

Mathematics, 31.03.2021 15:30

Mathematics, 31.03.2021 15:30

English, 31.03.2021 15:30

History, 31.03.2021 15:30

Engineering, 31.03.2021 15:30

Geography, 31.03.2021 15:30


Mathematics, 31.03.2021 15:30

Mathematics, 31.03.2021 15:30



Mathematics, 31.03.2021 15:30