Chemistry, 20.06.2020 04:57 sksksksksk1
The specific heat of mercury is 0.138 J/g Celsius. If 452 g of mercury at 85.0 Celsius are placed in 145 g of water at 23.0 Celsius, what will be the final temperature for both the mercury and the water?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
The specific heat of mercury is 0.138 J/g Celsius. If 452 g of mercury at 85.0 Celsius are placed in...
Questions

Mathematics, 05.04.2021 23:10

Mathematics, 05.04.2021 23:10


Mathematics, 05.04.2021 23:10





English, 05.04.2021 23:10

Social Studies, 05.04.2021 23:10

Biology, 05.04.2021 23:10



History, 05.04.2021 23:10

Mathematics, 05.04.2021 23:10



Mathematics, 05.04.2021 23:10

Chemistry, 05.04.2021 23:10

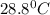
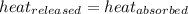
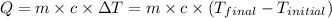
![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0690/6124/92a72.png)
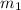 = mass of mercury= 452 g
= mass of mercury= 452 g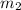 = mass of water = 145 g
= mass of water = 145 g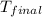 = final temperature = ?
= final temperature = ?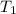 = temperature of mercury =
= temperature of mercury = 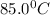
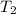 = temperature of water =
= temperature of water = 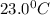
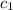 = specific heat of mercury =
= specific heat of mercury = 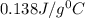
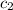 = specific heat of water=
= specific heat of water= 
![-[452\times 0.138\times (T_f-85.0)^0C]=145\times 4.184\times (T_f-23.0)^0C](/tpl/images/0690/6124/a875e.png)