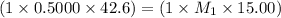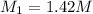Chemistry, 18.06.2020 15:57 umezinwachukwuebuka1
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the titration of 15.00 mL of an ammonia cleaner with 0.5000 M HCl, 42.6 mL of the titrant was required to reach the endpoint. What is the concentration of the NH3 in solution

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the t...
Questions

Mathematics, 01.09.2020 20:01

Mathematics, 01.09.2020 20:01

Physics, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01

Mathematics, 01.09.2020 20:01







Mathematics, 01.09.2020 20:01







Mathematics, 01.09.2020 20:01

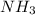 in solution is 1.42 M
in solution is 1.42 M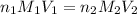
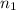 = basicity of
= basicity of 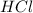 = 1
= 1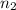 = acidity of
= acidity of 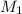 = concentration of
= concentration of 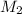 = concentration of
= concentration of 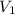 = volume of
= volume of 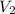 = volume of
= volume of