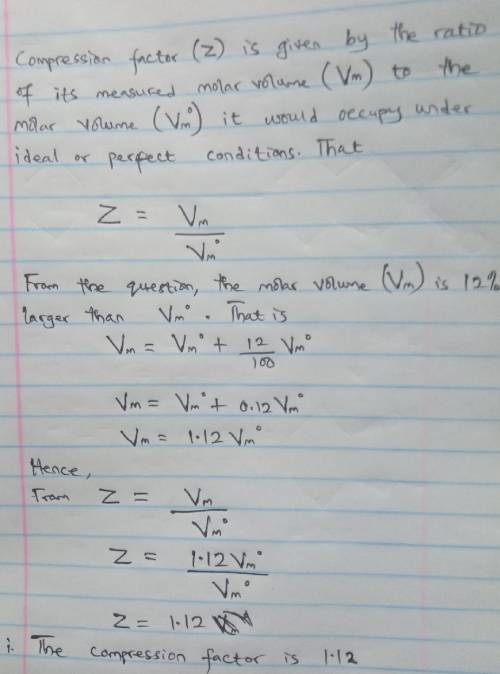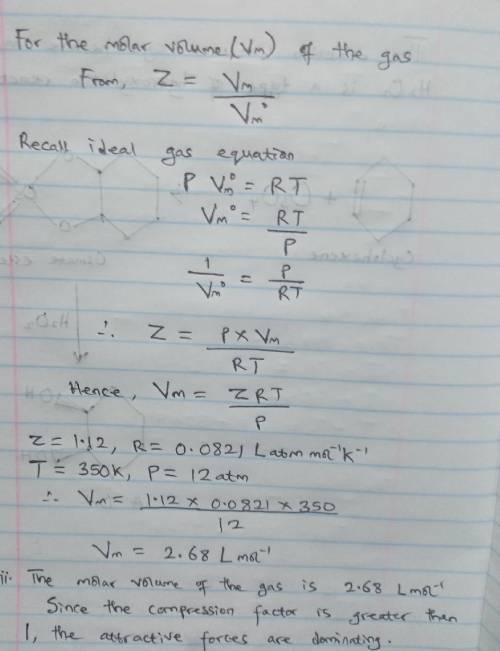A gas at 350 K and 12 atm has a molar volume 12 per cent larger than that calculated from the perfect gas law. Calculate (i) the compression factor under these conditions and (ii) the molar volume of gas. Which are dominating in the sample, the attractive or repulsive forces

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
A gas at 350 K and 12 atm has a molar volume 12 per cent larger than that calculated from the perfec...
Questions

English, 25.02.2021 01:00

English, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00

Social Studies, 25.02.2021 01:00


English, 25.02.2021 01:00


Biology, 25.02.2021 01:00

English, 25.02.2021 01:00






English, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00