
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+Pi⟶glucose-6-phosphate+H2 OATP+H2O⟶ADP+PiΔG=+13.8 kJ/molΔG=−30.5 kJ/mol reaction 1:glucose+Pi⟶glucose-6-phosphate+H2 OΔG=+13.8 kJ/molreaction 2:ATP+H2O⟶ADP+PiΔG=−30.5 kJ/mol Answer the four questions about the first step of glycolysis. Is reaction 2 spontaneous or nonspontaneous?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+...
Questions


Chemistry, 22.05.2021 09:30




Mathematics, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30

Computers and Technology, 22.05.2021 09:30

Biology, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30


Computers and Technology, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30

Mathematics, 22.05.2021 09:30



English, 22.05.2021 09:30

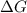 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol
glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol

