
Chemistry, 18.06.2020 07:57 joslynndiggs
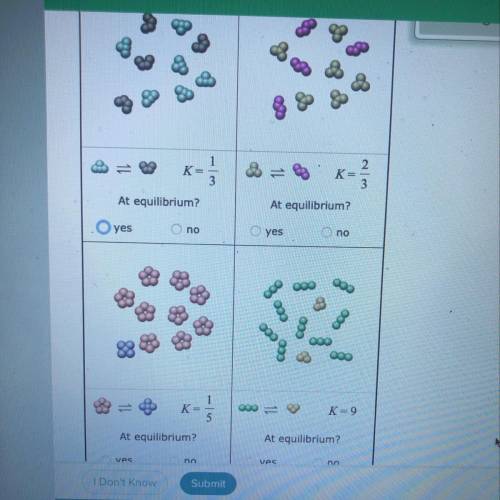
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are
not shown.)
The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion
equilibrium is shown below the sketch.
Decide whether each solution is at equilibrium.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

You know the right answer?
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that indi...
Questions











Chemistry, 16.04.2020 23:51

Biology, 16.04.2020 23:51




Engineering, 16.04.2020 23:51






