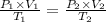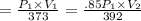Chemistry, 17.06.2020 19:57 deshawnnash53
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of - 161. °C. Suppose the temperature of a sample of methane gas is raised from 100.0 °C to 119.0 °C, and at the same time the pressure is decreased by 15.0%. increase Does the volume of the sample increase, decrease, or stay the same? decrease x 6 ? stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. 1%

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions


English, 26.10.2021 17:50



History, 26.10.2021 17:50

Mathematics, 26.10.2021 17:50








History, 26.10.2021 17:50

French, 26.10.2021 17:50


Social Studies, 26.10.2021 17:50


French, 26.10.2021 17:50