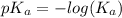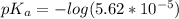Chemistry, 16.06.2020 19:57 Rachaeltice8810
Since some amino acids, such as Glu and His, can be viewed as weak acids or bases, the ratio of their protonated form to their deprotonated form is dependent on pH. Furthermore, you can use this knowledge to predict whether a given amino acid will exist predominantly in its protonated or deprotonated form at a given pH. To do this, it is easier to think about the pK, of the amino acid functional group. Recall from general chemistry that the pK, is equal to -log(K). Using the K, value for the Glu side chain, which is 5.62 x 10-5, calculate the pK, for the Glu side chain. pKa =

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
Since some amino acids, such as Glu and His, can be viewed as weak acids or bases, the ratio of thei...
Questions

Law, 21.07.2021 21:30




Mathematics, 21.07.2021 21:30


Mathematics, 21.07.2021 21:30

Mathematics, 21.07.2021 21:30

Mathematics, 21.07.2021 21:30




Law, 21.07.2021 21:30

Social Studies, 21.07.2021 21:30

English, 21.07.2021 21:30

Health, 21.07.2021 21:30


Mathematics, 21.07.2021 21:30


Biology, 21.07.2021 21:30

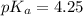
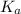 value of Glu is
value of Glu is 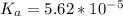
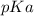 is mathematically represented as
is mathematically represented as