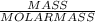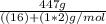Chemistry, 22.12.2019 02:31 jachecj3269
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor, if you burn 50.0g of hydrogen and produce 447g of water how much oxygen is reacted

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor, if...
Questions

Mathematics, 19.08.2019 01:50





Mathematics, 19.08.2019 01:50


Mathematics, 19.08.2019 01:50


History, 19.08.2019 01:50



Mathematics, 19.08.2019 01:50



Mathematics, 19.08.2019 01:50