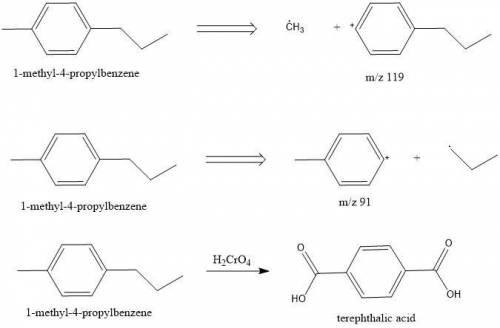
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Compound B also shows a less prominent peak at m/z 91. On vigorous oxidation with chromic acid, compound A is nonreactive while compound B yielded terephthalic acid.
From this information, deduce the structures of both compounds, and then draw the structure of B.
You do not have to consider stereochemistry
You do not have to explicitly draw H atoms

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Comp...
Questions

Computers and Technology, 23.11.2020 19:10


Mathematics, 23.11.2020 19:10



Mathematics, 23.11.2020 19:10


Social Studies, 23.11.2020 19:10


Social Studies, 23.11.2020 19:10

Mathematics, 23.11.2020 19:10

English, 23.11.2020 19:10


Arts, 23.11.2020 19:10

History, 23.11.2020 19:10

Mathematics, 23.11.2020 19:10

Mathematics, 23.11.2020 19:10

Mathematics, 23.11.2020 19:10


History, 23.11.2020 19:10