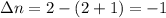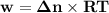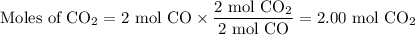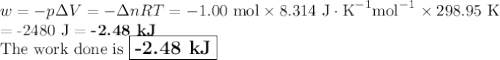Chemistry, 16.06.2020 02:57 estrellaalcantar16
Consider that you have a balloon containing 2.00 moles of CO and 1.00 mole of O2 which is in a room that has a temperature of 25.8oC and a pressure of 1.00 atm. Then, the following reaction occurs inside the balloon to completion: 2 CO(g) + O2(g) -> 2 CO2(g). Calculate the change in work due to the reaction occurring inside the balloon.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Consider that you have a balloon containing 2.00 moles of CO and 1.00 mole of O2 which is in a room...
Questions





Mathematics, 06.03.2020 22:19










Mathematics, 06.03.2020 22:21

English, 06.03.2020 22:21


Mathematics, 06.03.2020 22:21


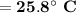
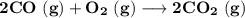
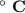 to
to 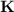 :
: 
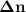 value:
value: