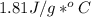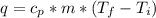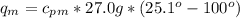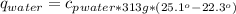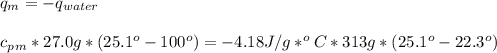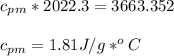Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
27.0g of an unknown metal at 100 degrees Celcius was dropped into a beaker containing 313g of water...
Questions

Mathematics, 18.06.2021 04:00


Mathematics, 18.06.2021 04:00

English, 18.06.2021 04:00




Mathematics, 18.06.2021 04:00


History, 18.06.2021 04:00



Mathematics, 18.06.2021 04:00




Social Studies, 18.06.2021 04:00

History, 18.06.2021 04:00