
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If the reaction starts with 2.15 mol of hydrogen bromide in 1.0 liter, and decomposes to 36.7%, what is the equilibrium constant of the decomposition of hydrogen bromide

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If...
Questions

Mathematics, 11.03.2021 17:30


Mathematics, 11.03.2021 17:30

History, 11.03.2021 17:30






Biology, 11.03.2021 17:30

Mathematics, 11.03.2021 17:30





Mathematics, 11.03.2021 17:30



Mathematics, 11.03.2021 17:30

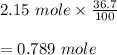
![K_c=\frac{[H_2][Br_2]}{[HBr]^2} \\\\=\frac{(0.395)(0.395)}{(1.361)^2} \\\\=\frac{0.156025}{1.852321} \\\\=0.084](/tpl/images/0685/5664/e5d29.png)


