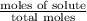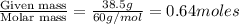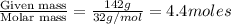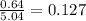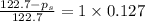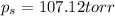Chemistry, 13.06.2020 23:57 reginaldlegette
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the non-volatile non-electrolye urea {CO(NH2)2} in 142 g of methanol. The vapor pressure of methanol at 298 K is 122.7 torr. Give your answer to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the n...
Questions




Mathematics, 20.01.2021 07:40


Mathematics, 20.01.2021 07:40



Chemistry, 20.01.2021 07:40

Social Studies, 20.01.2021 07:40

Chemistry, 20.01.2021 07:40



Social Studies, 20.01.2021 07:40

Mathematics, 20.01.2021 07:40




Mathematics, 20.01.2021 07:50

Mathematics, 20.01.2021 07:50

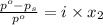
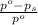 = relative lowering in vapor pressure
= relative lowering in vapor pressure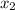 = mole fraction of solute =
= mole fraction of solute =