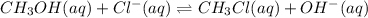Chemistry, 13.06.2020 22:57 donaji1024perez
Write the equilibrium constant expression for this reaction: CH3OH (aq)+Cl-(aq)-> CH3Cl(aq)+OH-(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Write the equilibrium constant expression for this reaction: CH3OH (aq)+Cl-(aq)-> CH3Cl(aq)+OH-(a...
Questions

Mathematics, 21.11.2020 05:40



Mathematics, 21.11.2020 05:40

Mathematics, 21.11.2020 05:40



Mathematics, 21.11.2020 05:40

Mathematics, 21.11.2020 05:40







Mathematics, 21.11.2020 05:40


English, 21.11.2020 05:40


Mathematics, 21.11.2020 05:40

![K_c=\frac{[CH_3Cl]\times [OH^-]}{[CH_3OH]\times [Cl^-]}](/tpl/images/0685/4559/fd6a4.png)