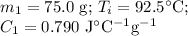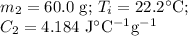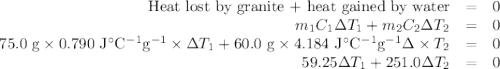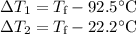Chemistry, 12.06.2020 21:57 dinosaur10
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC. Determine the final temperature (in oC) when they reach thermal equilibrium. Assume no heat loss to the surroundings. Specific heats: granite = 0.790 J/(goC), water = 4.184 J/goC

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC....
Questions

Business, 06.11.2020 21:40



Mathematics, 06.11.2020 21:40


Social Studies, 06.11.2020 21:40

Mathematics, 06.11.2020 21:40



English, 06.11.2020 21:40


Mathematics, 06.11.2020 21:40

Chemistry, 06.11.2020 21:40

Mathematics, 06.11.2020 21:40


Health, 06.11.2020 21:40

Mathematics, 06.11.2020 21:40


English, 06.11.2020 21:40