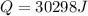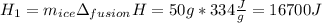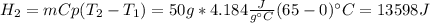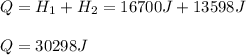Chemistry, 13.06.2020 09:57 xbeatdroperzx
How many joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C? heat of fusion of ice = 334 J/g specific heat of water = 4.184 J/g°C specific heat of ice = 2.03 J/g°C heat of vaporization of water = 2260J/g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
How many joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C? heat...
Questions



English, 02.10.2020 15:01

Health, 02.10.2020 15:01

Business, 02.10.2020 15:01

Business, 02.10.2020 15:01

History, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01



Mathematics, 02.10.2020 15:01



Mathematics, 02.10.2020 15:01


Medicine, 02.10.2020 15:01