Chemistry, 12.06.2020 12:57 milkshakegrande101
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has been heated to 115.0°C. The metal heats the water so that both the metal
and the water reach a final temperature of 29.3°C. Calculate the specific heat of the
metal.


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has b...
Questions

Mathematics, 02.08.2019 18:40


English, 02.08.2019 18:40


Mathematics, 02.08.2019 18:40

Mathematics, 02.08.2019 18:40

Physics, 02.08.2019 18:40


Geography, 02.08.2019 18:40

English, 02.08.2019 18:40

History, 02.08.2019 18:40

Physics, 02.08.2019 18:40


English, 02.08.2019 18:40


English, 02.08.2019 18:40

History, 02.08.2019 18:50



Chemistry, 02.08.2019 18:50

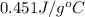
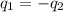
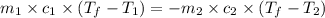
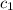 = specific heat of metal = ?
= specific heat of metal = ?
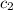 = specific heat of water =
= specific heat of water = 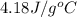
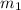 = mass of metal = 50.0 g
= mass of metal = 50.0 g
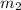 = mass of water = 125 g
= mass of water = 125 g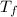 = final temperature of mixture =
= final temperature of mixture = 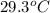
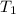 = initial temperature of metal =
= initial temperature of metal = 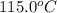
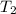 = initial temperature of water =
= initial temperature of water = 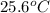
![(50.0g)\times c_1\times (29.3-115.0)^oC=-[(125g)\times 4.18J/g^oC\times (29.3-25.6)^oC]](/tpl/images/0684/0377/2c651.png)