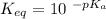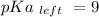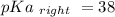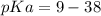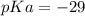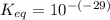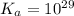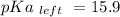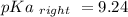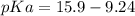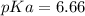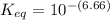Chemistry, 12.06.2020 04:57 katekayrodriguez10
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the left as written. (You may enter your answer in scientific notation, e. g. 1.0*10^-9. Enter your answer to two significant figures.) Reaction 1: + + pKa = 9 pKa = 38 Keq = Equilibrium position = Reaction 2: + + pKa = 35 pKa = 25 Keq = Equilibrium position =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the lef...
Questions

Mathematics, 15.01.2021 07:20


Mathematics, 15.01.2021 07:20



Chemistry, 15.01.2021 07:20


English, 15.01.2021 07:20



Arts, 15.01.2021 07:20

Computers and Technology, 15.01.2021 07:20

Mathematics, 15.01.2021 07:20



Biology, 15.01.2021 07:20

Mathematics, 15.01.2021 07:20


English, 15.01.2021 07:20

Mathematics, 15.01.2021 07:20

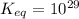
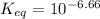
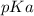 is mathematically evaluated as
is mathematically evaluated as 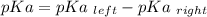
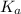 is mathematically evaluated as
is mathematically evaluated as