Chemistry, 12.06.2020 02:57 lilinicholeb
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be needed to burn 36.1 g of B2H6 (Molar mass = 27.67g/mol)? g
Include the correct number of significant figures in your final answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be n...
Questions

Mathematics, 20.07.2019 09:30

History, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30


Mathematics, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30




Geography, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30




Mathematics, 20.07.2019 09:30

Physics, 20.07.2019 09:30

Chemistry, 20.07.2019 09:30

Geography, 20.07.2019 09:30

History, 20.07.2019 09:30

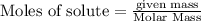
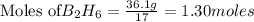
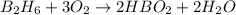
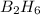 require = 3 moles of
require = 3 moles of 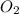
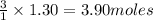 of
of