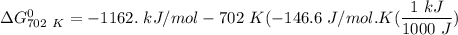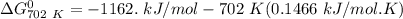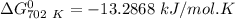Consider the following reaction:
2NO(g)+O2(g)→2NO2(g)
Estimate ΔG∘ for this reaction at each of the following temperatures and predict whether or not the reaction will be spontaneous. (Assume that ΔH∘ and ΔS∘ do not change too much within the give temperature range.) I need to find the temperature are 298K and 702K. For 298K It is simple because at standard temperature
ΔG∘ = DG(products)- DG(reactants).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Consider the following reaction:
2NO(g)+O2(g)→2NO2(g)
Estimate ΔG∘ for this reaction at each...
Estimate ΔG∘ for this reaction at each...
Questions

Geography, 02.09.2019 01:30

Spanish, 02.09.2019 01:30


Mathematics, 02.09.2019 01:30




English, 02.09.2019 01:30


Social Studies, 02.09.2019 01:30







History, 02.09.2019 01:30



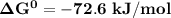 ; as such the reaction is said to be spontaneous since the value of
; as such the reaction is said to be spontaneous since the value of 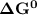 is negative.
is negative.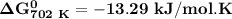 and the reaction is spontaneous
and the reaction is spontaneous
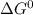 ;
;![\Delta G^0 = [2(\Delta G^0_{NO_{2(g)}}] - [1(\Delta G^0_{O_{2(g)}})+ 2(\Delta G^0_{NO_{g}})]](/tpl/images/0683/6875/905f6.png)

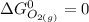
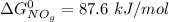
![\Delta G^0 = [2(51.2 \ kJ/mol}] - [1(0)+ 2(87.6 \ kJ/mol})]](/tpl/images/0683/6875/34022.png)
![\Delta G^0 = [102.4 \ kJ/mol}] - [175.2 \ kJ/mol})]](/tpl/images/0683/6875/a6424.png)
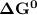 of the reaction when the temperature is 702 K is:
of the reaction when the temperature is 702 K is: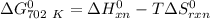
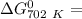 Gibbs free energy of the reaction at 702 K
Gibbs free energy of the reaction at 702 K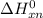 = standard enthalpy of the reaction = -116.2 kJ/mol
= standard enthalpy of the reaction = -116.2 kJ/mol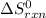 = standard entropy of the reaction = -146.6 J/mol/K
= standard entropy of the reaction = -146.6 J/mol/K