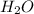Chemistry, 11.06.2020 10:57 shazanah95
Identify the Lewis acids and Lewis bases in the following reactions:
1. H+ + OH- <-> H2O Lewis acid: Lewis base:
2. Cl- + BCl3 <-> BCl4- Lewis acid: Lewis base:
3. K+ + 6H2O <-> K(H2O)6+ Lewis acid: Lewis base:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1

You know the right answer?
Identify the Lewis acids and Lewis bases in the following reactions:
1. H+ + OH- <-> H2O Lewi...
Questions

Mathematics, 06.05.2021 14:00




Mathematics, 06.05.2021 14:00

Geography, 06.05.2021 14:00



English, 06.05.2021 14:00


Computers and Technology, 06.05.2021 14:00

Social Studies, 06.05.2021 14:00


Health, 06.05.2021 14:00

World Languages, 06.05.2021 14:00




English, 06.05.2021 14:00

SAT, 06.05.2021 14:00

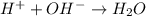 Lewis acid :
Lewis acid : 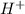 , Lewis base :
, Lewis base : 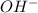
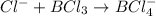 Lewis acid :
Lewis acid : 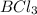 , Lewis base :
, Lewis base : 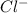
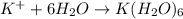 Lewis acid :
Lewis acid : 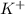 , Lewis base :
, Lewis base :