

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
According to the ideal gas law, what happens to the volume of a gas when the
temperature doubles (a...
Questions

English, 19.09.2019 16:30




Computers and Technology, 19.09.2019 16:30

Social Studies, 19.09.2019 16:30


Mathematics, 19.09.2019 16:30



Social Studies, 19.09.2019 16:30

Mathematics, 19.09.2019 16:30

Computers and Technology, 19.09.2019 16:30



Physics, 19.09.2019 16:30

Mathematics, 19.09.2019 16:30

Chemistry, 19.09.2019 16:30

History, 19.09.2019 16:30

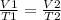 And volume and temperature are directly correlated.
And volume and temperature are directly correlated.

