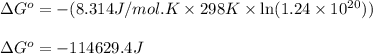In the activity, click on the Keq and ΔG∘ quantities to observe how they are related. Calculate ΔG∘using this relationship and the equilibrium constant (Keq) obtained in Part A at T=298K:Keq=1.24×1020Express the Gibbs free energy (ΔG∘) in joules to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
In the activity, click on the Keq and ΔG∘ quantities to observe how they are related. Calculate ΔG∘u...
Questions

English, 22.02.2020 01:29


Mathematics, 22.02.2020 01:29

Physics, 22.02.2020 01:30


Physics, 22.02.2020 01:30



History, 22.02.2020 01:30


History, 22.02.2020 01:30



Mathematics, 22.02.2020 01:31



English, 22.02.2020 01:31

Mathematics, 22.02.2020 01:31

History, 22.02.2020 01:31

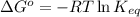
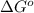 = Gibbs free energy of the reaction = ?
= Gibbs free energy of the reaction = ?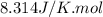
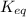 = equilibrium constant of the reaction =
= equilibrium constant of the reaction =