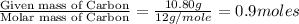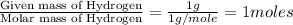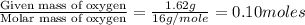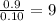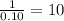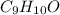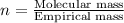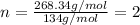Chemistry, 10.06.2020 19:57 calhountoiyonou0gjb
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.61 g CO2 and 9.01 g H2O. The molar mass of equilin is 268.34 g/mol. Find its molecular formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon,...
Questions



Mathematics, 05.07.2019 20:30



Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30


Business, 05.07.2019 20:30


Mathematics, 05.07.2019 20:30




Chemistry, 05.07.2019 20:30

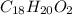
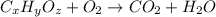
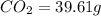
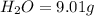
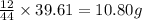 of carbon will be contained.
of carbon will be contained.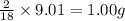 of hydrogen will be contained.
of hydrogen will be contained.