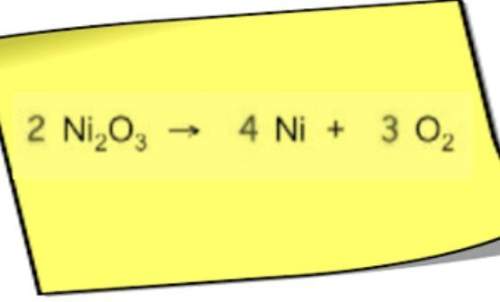
Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2


Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
In the reaction: ca+2hcl->cacl2+h2 20.0g of calcium was created with excess hydrochloric acid and...
Questions

Mathematics, 05.05.2021 20:50

History, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50



Physics, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50

Spanish, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50


Mathematics, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50


Computers and Technology, 05.05.2021 20:50