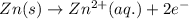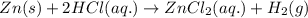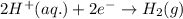Chemistry, 10.06.2020 03:57 willveloz4
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce zincil) choride (ZnCl2) and
hydrogen gas (H2) according to the following reaction:
Zn(s) + 2HCl(aq) > ZnCl2(aq) + H2(g)
Part A
How many moles of ZnCl2 are produced?
moles =
Part B
How many grams of H2 are produced?
grams =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce zincil) choride (ZnCl...
Questions


Biology, 18.10.2021 22:50

Mathematics, 18.10.2021 22:50

Social Studies, 18.10.2021 22:50




Mathematics, 18.10.2021 22:50

Business, 18.10.2021 22:50

World Languages, 18.10.2021 22:50

Biology, 18.10.2021 22:50


Mathematics, 18.10.2021 22:50

Chemistry, 18.10.2021 22:50

Health, 18.10.2021 22:50



Physics, 18.10.2021 22:50